Plutonium (Pu) Element: History, Properties, Uses, Hazards

Plutonium, which belongs to the actinide class of the Periodic Table, has an atomic number of 94. The symbol “Pu” is used to represent it. The metal appears silvery initially, but when it oxidizes slightly, it tarnishes yellow. It is an element that has been synthesized. Similar to other elements in the actinide class, plutonium exhibits significant radioactivity. It is an explosive element found in almost all current nuclear weapons. American chemists Glenn T. Seaborg, Joseph W. Kennedy, and Arthur C. Wahl discovered plutonium-238 in 1941. They created it by bombarding uranium-238 with deuterons in a 152-cm (60-inch) cyclotron in Berkeley, California.
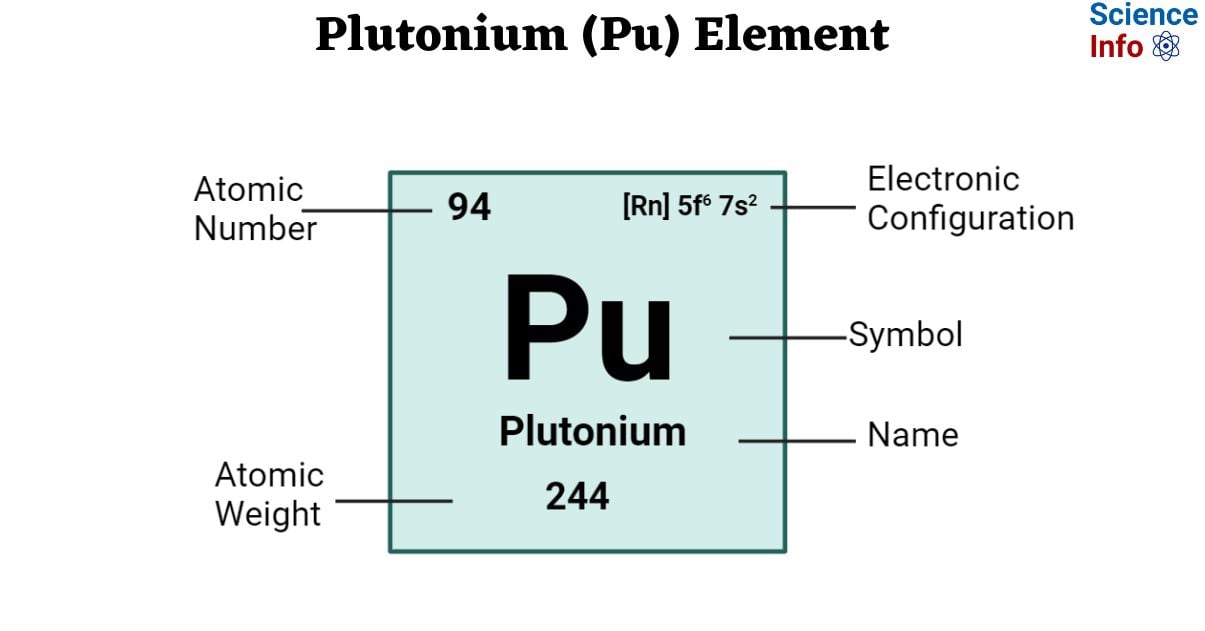
Table of Contents
Interesting Science Videos
History of Plutonium
- Glenn Seaborg, Edwin McMillan, Joseph Kennedy, and Arthur Wahl created plutonium for the first time in 1940. It was the second synthesized trans-uranium element from the actinide series to be identified.
- During the very same year, it was discovered that slow neutrons cause plutonium-239 to fission. The fission process produces more neutrons, which might cause a nuclear chain reaction.
- The metal was later discovered naturally in trace amounts as a decay product in uranium ores.
- Since it was discovered shortly after Neptunium, it was given the name Pluto. Pluto was envisioned to be the planet to follow after Neptune.
- In 1942, Burris Cunningham and Louis Werner extracted a microgram of pure plutonium-239 compound (plutonium IV iodate) from the University of Chicago’s Metallurgical Laboratory.
- This was the first time a compound of an artificially synthesized element has been created in a detectable quantity, enabling for extensive investigations of its properties.
Occurrence of Plutonium
- Trace amounts of plutonium can be discovered in uranium ores naturally. It is manufactured commercially in considerable quantities from 238 U in nuclear reactors.
- Further on, traces of plutonium have been discovered in uranium ores, where it is naturally created by neutron radiation rather than being primordial.
- In 1972, a high concentration of plutonium was identified in the natural nuclear fission reactor at Oklo, Gabon. Since 1945, nuclear explosions have emitted approximately 7700 kg of elements onto the Earth.
- Plutonium has 17 isotopes with half-lives and masses ranging from 227 to 248. None are stable.
- Every isotope of plutonium is radioactive.
Elemental Properties of Plutonium
| Electronic Configuration | [Rn] 5f 6 7s 2 |
| Atomic Number | 94 |
| Atomic Weight | 244 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | Actinides, 7, f-block |
| Density | 19.84 g/cm 3 at 20 °C |
| Ionic radius | unknown |
| Van der Waals radius | – |
| Electron shells | 2, 8, 18, 32, 24, 8, 2 |
| Electrons | 94 |
| Protons | 94 |
| Neutrons | 150 (Varies with isotopes) |
Isotopic Information of Plutonium
- Twenty-one plutonium radioisotopes have been identified.
- Pu-244 has the longest half-life at 80.8 million years, followed by Pu-242 at 373,300 years and Pu-239 at 24,110 years.
- The rest of the other radioactive isotopes have half-lives of fewer than 7,000 years.
- This element has eight meta-states as well, but none are particularly stable (all have half-lives of less than one second).
- Plutonium isotope exhibit atomic weights ranging from 228.0387 (Pu-228) to 247.074 (Pu-247).
- Before the most stable isotope, Pu-244, the principal decay processes include spontaneous fission and alpha emission, followed by beta emission.
Physical Properties of Plutonium
- Plutonium exhibits exceptionally high resistivity at room temperature, a property that remains pronounced even at lower temperatures.
- Upon melting, plutonium experiences a 2.5% increase in density, yet it possesses notably high surface tension and viscosity compared to other metals at its melting point. This actinide metal boasts a bright silvery appearance.
- Plutonium readily forms intermediate compounds and alloys with a wide range of metals.
- Transuranium elements such as Neptunium, Americium, and Curium, in addition to Plutonium, pose a radiological hazard, necessitating careful handling with precaution.
- Plutonium has an atomic mass of 244.
- The melting point of plutonium is 640°C, while its boiling point is 3228°C.
- At 20°C, the density of plutonium is 19840 in S.I. units.
- Plutonium’s electrical resistance is exceptionally high at room temperature, and this resistance escalates as the temperature decreases.
- Plutonium can exist in seven different allotropic forms.
- To date, nearly 20 radioactive isotopes of plutonium have been characterized.

| Physical Property | Plutonium |
| Color/Physical Appearance | Bright silvery with a metallic luster |
| Melting Point/Freezing Point | 640°C (1184°F, 913 K) |
| Boiling Point | 3228°C (5842°F, 3501 K) |
| Density | 19.84 g/cm³ |
| Flammability | Little fragments can spontaneously ignite in air |
| State of Matter at Room Temperature | Solid |
Chemical Properties of Plutonium
- Plutonium showcases six allotropes, with the alpha form prevailing at room temperature.
- It possesses the highest electrical resistance among metallic elements.
- Plutonium exhibits a range of oxidation states from +3 to +6.
- Plutonium dissolves in acids and can adopt four oxidation states in aqueous solutions, each associated with distinctive ion colors:
Pu 3+ : Blue-lavender
Pu 4+ : Yellow-brown
PuO2 2+ : Yellow or pink-orange
Chemical Reactions of Plutonium
Reaction of plutonium with acids
- Plutonium is dissolved by hydrochloric, hydroiodic, and perchlor acid.
2 Pu (s) + 6 H + (aq) → 2 Pu 3+ (aq) + 3 H2(g)
Reaction of plutonium with air
- In air, plutonium is covered by a thin layer of plutonium oxide.
Uses of Plutonium
Chemical Industries and Nuclear Applications: Plutonium reacts with oxygen, carbon, and fluorine, producing compounds used directly or as intermediates in the nuclear industry.
Role in Nuclear Weapons: The isotope plutonium-239, which is easily fissile and abundant, is used extensively in the manufacturing of nuclear weapons.
Power Generation in Spacecraft: The plutonium isotope 238Pu, which has an 87-year half-life and emits alpha radiation, is used in RTGs (radioisotope thermoelectric generators) to power long-term space missions such as Cassini and Galileo.
Long-Lasting Power Source: Plutonium-238’s radioactive decay, which emits alpha radiation with little gamma-ray emission, is used to generate heat for thermoelectric and thermionic devices that are compact, lightweight, and long-lasting.
Research applications: Plutonium-242 and plutonium-244 have use in chemical and metallurgical research.
Space exploration and artificial cardiac pacemakers: Plutonium-238 is used as a power source in space probes such as the Voyager spacecraft and on the Apollo-14 lunar mission. It is also used in artificial heart pacemakers, which reduces the need for further surgery.
Fuel in breeder reactors: Plutonium carbides (PuC, PuC2, and Pu2C3) serve as fuel in breeder reactors, contributing to nuclear energy production.
Thermoelectric Generator Applications: Plutonium’s capacity to convert heat into electricity makes it ideal for thermoelectric generator applications, especially in space missions and medical devices such as artificial pacemakers. Plutonium-238 is favored for these uses due to its low radiation profile.
Nuclear Power Plants and Weapon Production: Plutonium-239, which can undergo nuclear fission, is used as fuel in nuclear power plants and is an important component in the creation of nuclear weapons, providing a powerful explosive equivalent to more than 10,000 tons of chemical explosive.
Contributions to Nuclear Power Development: Plutonium has played an important part in the advancement of nuclear power, providing energy for space missions such as the Mars Curiosity Rover and the New Horizons spacecraft on their way to Pluto.
Health Effects of Plutonium
- Plutonium, which is sometimes portrayed falsely as the most dangerous substance, has no evidence tying it to human deaths. Natural radium and certain organic toxins, such as botulinum toxin, are more toxic than plutonium.
- Alpha radiation emitted by plutonium does not penetrate the skin but poses risks when inhaled or ingested. Minute particles of plutonium, even in microgram quantities, can lead to lung cancer.
- Ingesting or inhaling larger amounts of plutonium may cause acute radiation poisoning and death. Despite this, no confirmed human deaths have resulted from plutonium exposure, and many individuals carry measurable amounts of plutonium in their bodies.
- Plutonium, which is released into the atmosphere during nuclear weapon testing and accidents, eventually falls to Earth, leading to soil contamination.
- While human exposure to plutonium is unlikely, unintentional exposures during usage, transportation, or disposal are possible.
- Plutonium, once inhaled, may remain in the lungs or move to bones and organs, exposing body tissues to continuous radiation for an extended period of time and potentially leading to cancer within a few years.
- Plutonium may impair the body’s ability to fight sickness, and its radioactivity may contribute to reproductive failure.
- Plutonium-239, a toxic carcinogen and nuclear weapon component, poses a significant risk due to its difficulty detecting outside secure facilities.
- Plutonium-239 alpha radiation, which preferentially deposits in soft tissues, bone surfaces, and bone marrow, produces greater biological damage than other forms of radiation, increasing the likelihood of cancer development.
- External exposure to plutonium-239, particularly in its typical oxide form, provides little risk due to alpha particles depositing energy in the outer skin layer.
- The primary health danger is from inhalation, particularly when dealing with insoluble plutonium-239 oxide. Another worry is absorption through cuts and abrasions, but eating has a reduced danger in general.
- Larger plutonium particles cause less biological damage and pose a lower illness risk than smaller particles. Smaller particles inhaled can become lodged in the lungs and irradiate the surrounding tissue.
- Other plutonium isotopes that release alpha radiation, such as plutonium-238, provide similar health consequences per unit of radioactivity. However, radioactivity per unit weight varies by isotope, with plutonium-238 being substantially more radioactive than plutonium-239.
Environmental Effects of Plutonium
- Plutonium may end up in surface water as a result of accidentally nuclear waste leaks and disposal.
- Fallout from nuclear weapons testing can contaminate soil with plutonium. Plutonium gradually moves downward via soil until it reaches groundwater and hence contaminates water as well.
- Plants absorb little amounts of plutonium, which are inadequate for biomagnification in the food chain or accumulation in animal bodies.
- Accidental discharges and disposal techniques can have an influence on aquatic ecosystems, possibly threatening marine life and biodiversity.
- The long-term impacts of plutonium contamination in soil and water, particularly with regard to ecosystem dynamics, necessitate constant monitoring and investigation.
Video on Plutonium

References
- https://education.jlab.org/itselemental/ele094.html
- https://testbook.com/chemistry/plutonium
- https://www.britannica.com/science/argon-chemical-element
- W. M. Haynes, ed., CRC Handbook of Chemistry and Physics, CRC Press/Taylor and Francis, Boca Raton, FL, 95th Edition, Internet Version 2015, accessed December 2014.
Tables of Physical & Chemical Constants, Kaye & Laby Online, 16th edition, 1995. Version 1.0 (2005), accessed December 2014.
J. S. Coursey, D. J. Schwab, J. J. Tsai, and R. A. Dragoset, Atomic Weights and Isotopic Compositions (version 4.1), 2015, National Institute of Standards and Technology, Gaithersburg, MD, accessed November 2016.
T. L. Cottrell, The Strengths of Chemical Bonds, Butterworth, London, 1954.
- https://coto2.wordpress.com/2011/03/29/health-effects-of-plutonium/
- BARNABY, FRANK. “The Radiological Hazards of Plutonium.” Medicine, Conflict and Survival 13, no. 3 (1997): 195–206. http://www.jstor.org/stable/45354931.
- Toxicological Profile for Plutonium. Atlanta (GA): Agency for Toxic Substances and Disease Registry (US); 2010 Nov. 3, HEALTH EFFECTS. Available from: https://www.ncbi.nlm.nih.gov/books/NBK599402/
- https://www.lenntech.com/periodic/elements/pu.htm
- https://byjus.com/chemistry/plutonium/
About Author





